Silanes
Handling Precautions
Quality and Safety
- Silanes are identified as No. 4 fire hazards under Japanese fire fighting law. They should be handled with care and kept away from flames. Please refer to our SDS, and contact our sales department for details.
- While working with chlorosilanes, wear gloves and goggles for protection from contamination, because chlorosilanes are very reactive with moisture yielding hydrochloric acid gas. In case of the contact with the skin or membranes, flush with running water and consult with a doctor immediately. Chlorosilanes also should be handled with adequate ventilation.
- Silanes that consisting of Si-H will yield hydrogen gas when contacted with alkaline or under the existence of platinum catalyst by the reaction with chemical composite having activated hydorogen such as alcohol, amine, etc. These silanes should be handled with care to keep away from flame and the pressure resistance of the reactive containers and containers for storage should be watched carefully.
- Alkoxysilanes should be handled with care to avoid contact with the eyes and skin and gloves and goggles are recommended while using these products. In case of contact, the eyes or membranes should be flushed with running water and a doctor should be consulted. Use should be in a location with adequate ventilation.
- Please refer to the following articles for guidelines to chlorosilane emergency responses, in case of a large-scale operation, an electrical short circuit, fire hazards, ect.
ASTM Manual Series:MNL33 "Manual on Chlorosilane Emergency Response Guidelines" (John T. Higgins, Wilmuth E. Hubbs, Robert A. Kayser, Paul Kremer, Stephen W. MacMahan, Lee Patricc and Micheal Strong, editors 1998)
Chemical Structure
Shin-Etsu functional silanes are silicone organic monomers with the structures shown below:
R=organic group (-H, -CH3, -C6H6, -CnH2n+1)
X=hydrolysis group (-Cl, -OCH3, -OC2H5)
Reaction
The silanes described in this web site are reactive to compounds with activated hydrogens such as water, alcohol or amines.
Chlorosilanes
The reactions of chlorosilanes are shown below:
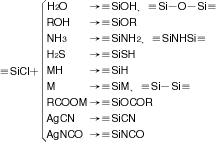
M: alkaline metals, alkaline earth metal
Alkoxysilanes (methoxysilanes, ethoxysilane), silazanes
Alkoxysilanes and silazanes are usually reactive to compounds with activated hydrogen such as water and alcohol. Unlike chlorosilanes, they will not yield hydrogen chloride and are easy to handle.
Applications
Shin-Etsu silanes are widely used as silylating agents for formulating pharmaceuticals or agricultural chemicals, and modifiers for paints or resins.
| Applications | Silanes | ||
|---|---|---|---|
| Chlorosilane | Alkoxysilane | Silazane | |
| Silylating agents for pharmaceuticals or agricultural chemicals |
|||
| Primers | |||
| Surface modifiers for non-organic materials |
|||
| Paint Modifiers | |||
| Raw materials for ceramics | |||
| Electronics materials | |||
| Resin modifiers | |||
![]() : Recommended to use
: Recommended to use ![]() : Can be used
: Can be used
